SYNOPSIS: 2nd Boston Paris Biotechnology Summit, 3 June 2018
June 18, 2018
The 2nd Boston/Paris Biotechnology Summit was held on Sunday 3 June 2018 at the Boston Exchange Conference Center, just prior to the 2018 BIO Convention held in Boston MA.
The Boston – Paris Biotechnology Summit is an exclusive, trans-Atlantic bridge designed to foster innovative synergies between biotech and pharma companies, healthcare-focused cities and regional clusters, and institutional, philanthropic and strategic investors. The Summits is designed to spark projects, financing and strategic deals to solve unmet medical needs and improve patient lives globally. The 2018 Summit was managed by an Organizing Committee with support from an Expert Advisory Group.
Over 200 attendees registered for the Summit, representing 135 organizations based in Atlanta, Bordeaux, Boston/Cambridge/MA, Chicago, Lille, Limoges, London, Lyon, Miami, Nancy, Montreal, New York/Greater NY Area, Toulouse, Vancouver, etc. We counted close to 100 CEOs, decision makers or equivalent level in attendance.
The BOSTON-PARIS Biotechnology Summit is an independent initiative supported by a panel of professionals from the biotech industry on both sides of the Atlantic. It seeks to establish relationships between Boston and French, European and international players and to stimulate and sustain synergies in investment, science, “market access” and regulatory practices. The efficient transfer of French biotech companies to the US, particularly in the Greater Boston area – the industry’s “Worldwide Hub” – demonstrates how these firms have matured in the life sciences. The aim of the Boston-based 2018 Organizing Committee (Youssef Bennani PhD, Soheila Gharakhanian, Shahin Gharakhanian MD, DPH, Frederic E “Rick” Pierce II,) was thus establish “Win-Win” relationships in both directions.
The first Summit had been held in May 2017 at the Institut Pasteur in Paris, France. In two years, the Summit Co-Founders and Organizing Committees have achieved the following milestones:
1) The Summit has become the premiere French-American biotechnology event held on a single day.
2) It offers wide-ranging knowledge and technical content (clinical trials, KOL relationships, regulatory strategy, Boston Ecosystem, market entry strategies, etc.).
3) Vision, strategic awareness and networking focus for start-ups.
Media coverage of the 2018 event included two press releases in Boston (30 May) and Paris (17 April), as well as Linked In postings and cover-page headlines in the widely read and referenced French news magazine “Biotech Finances” [No. 813, dated 28 May 2018].
The 2018 program and content were determined after research and feedback from the biotechnology community. This year’s central theme was “From the Promise of Raising Capital to the Reality of Clinical Trials”.
The Organizing Committee, (Soheila Gharakhanian, Shahin Gharakhanian MD, Rick Pierce, speaking) laid down the format for the event, and official representatives from the City of Boston (Krista Zelatores, Chief of Staff for Economic Development to the Mayor of Boston) and the Consulate General of France in Boston (Valery Freland, Consul General) opened the Summit.
Summary Box 1 – Summit Opening

The 2018 Vision on Science and Business Strategy lectures were given by the three leaders in the field:
- David Meek, CEO Ipsen, Paris FR & Cambridge MA: Trans-Atlantic Synergy: My Vision and Our Practice at Ipsen
- Robert G. Urban, PhD, Global Head, Johnson & Johnson Innovation, Boston, MA: Healthcare Innovations, Trends, Challenges and Aspirations
- Marc Murcko, PhD, Drug Hunter, Pharmaceutical Executive, Mentor, Disruptive Technology Advocate, Sr. Lecturer at MIT, CSO Relay Therapeutics, Cambridge MA: How I Hunt Drugs in Boston.
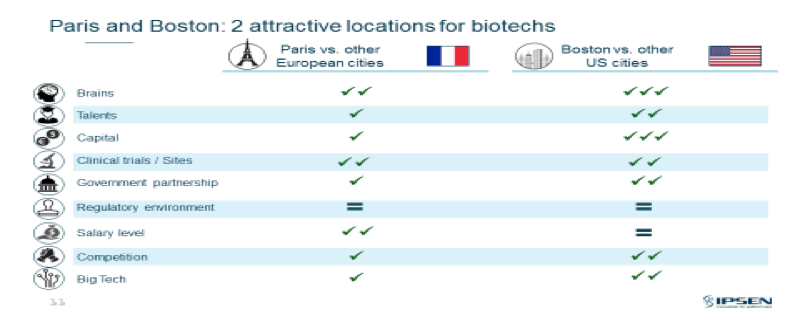
Box 1 summarizes the key points raised by David Meek in his presentation on international ecosystems:
Summary Box 1 –
Biotechnology ecosystems and criteria/characteristics
[Slide courtesy of David Meek, Ipsen]
Dr. Robert Urban then explored the concept of innovation and how organizations can sustain it. Robert G. Urban, PhD is Global Head of Johnson & Johnson Innovation; he joined the Johnson & Johnson Family of Companies from the Massachusetts Institute of Technology, where he was the founding Executive Director of the Koch Institute for Integrative Cancer Research. In this role, Robert worked to build the Koch Institute into a new standard for interdisciplinary disease-focused research via an expanding, highly-effective, relationship network with other academic oncology centers, industrial partners, cancer-focused philanthropists and investors. During Robert’s tenure, the Koch Institute launched 17 start-up companies and its technology was the source of more than 50 out-licensing transactions.
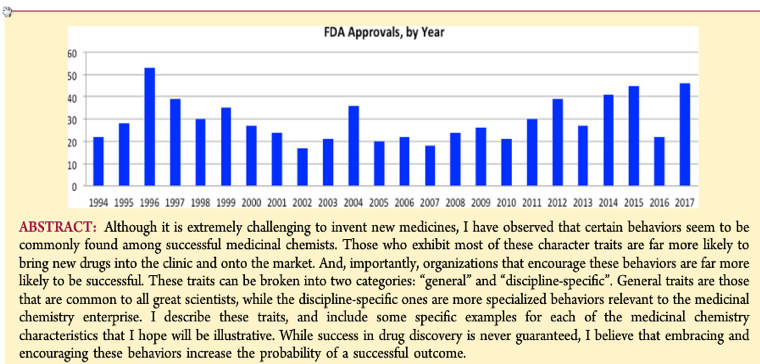
Box 2 summarizes some of the core points raised by Mark Murcko, PhD.
Summary Box 3 –Murcko M, J. Med. Chem., Publication Date:
May 10, 2018 (Perspective) DOI: 10.1021/acs.jmedchem.7b01445
US Roadmap 1 involved a panel focused on Academic Thought Leaders, Clinical Trials and Collaboration Panel:
- Academic, start-up and major pharma viewpoints
- The “Boston Model” in R&D, Clinical Development?
- The role of academic experts in biotech start-ups?
Panelists:
- Professor Kenneth Kaitin, PhD, Director CSDD: Center for Study of Drug Development, TUFTS University, Boston MA (Chair)
- Camilla “Cami” Graham, MD, MPH BIDC Medical Center, Harvard Medical School, Boston MA, Trek Therapeutics, Cambridge MA
- Joseph Merola, MD, MMSc, Director of Clinical Trials and Center for Skin and Related Musculoskeletal Diseases, BWH, Harvard Medical School, Boston MA
- Robert, G Urban, PhD, Global Head, Johnson & Johnson
Professor K. Kaitin summarized the characteristics of a dynamic regional cluster based on experience in MA as follows (alphabetical order):
- A culture of creativity, open-mindedness and collaboration
- Innovative small pharma/biotech companies and start-ups
- Investor and VC base
- Large Pharma, significant presence
- Local government commitment and support
- Universities, medical centers, teaching hospitals.
All participants engaged in very intensive networking during the lunch period.
US ROADMAP 2 involved a panel focused on Regulatory Strategy:
- FDA Update Circa 2018
- Pragmatic EMA/FDA comparisons – The need for strategic regulatory operations
Panelists:
- Moderator: Emmanuelle Trombe, partner at McDermott, Will & Emery (MWE), Paris, FR
- Speakers: Mark De Rosch, PhD Senior Vice President, Regulatory Affairs and Quality Assurance, Nightstar Therapeutics, Lexington MA, Veleka R. Peeples-Dyer, Co-Leader, FDA Practice, Life Sciences Industry Group, McDermott, Will & Emery.
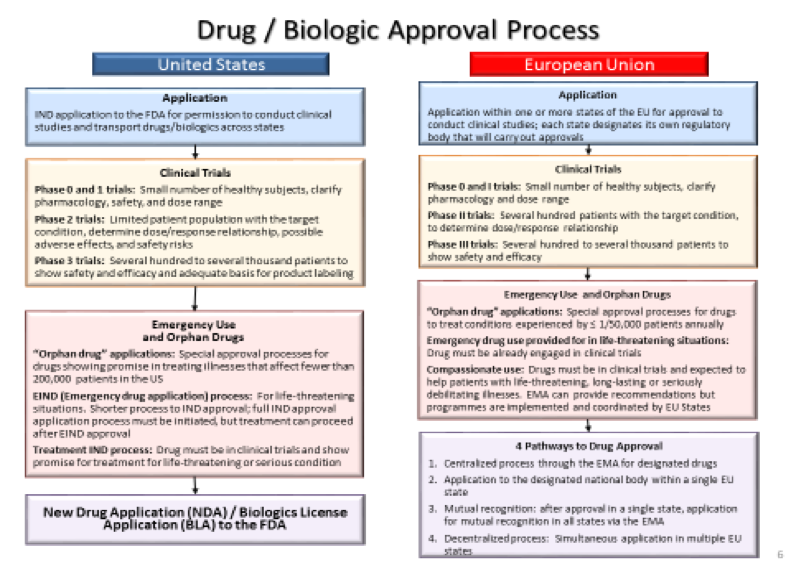
Summary Box 4– – Drug & Biologic Approval Processes in the US and EU
[Slide courtesy of McDermott Will Emery & M De Rosch]
GLOBAL ROADMAP 3 (Panel #3) focused on a US Entry & Funding Forum: Selected Company Pitches to a Boston Panel of Executives, Experts & Investors
Panelists:
- Pravin Chaturvedi, PhD (Chair), CEO and Co-Founder, Oceanyx Pharmaceuticals, SAB (Scientific Advisory Board), Napo Pharmaceuticals, Woburn MA
- Cynthia “Cindy” Lander, PhD, CEO Moerae Matrix. Partner, Nascent Enterprises, Venture Catalyst Partnership, Greater New York Area, NY
- Nicola La Monica, PhD, Senior Director, Infectious Disease Scientific Innovation at Janssen, Pharmaceutical Companies of Johnson & Johnson, Boston MA
- Michael Nowak, MBA, Managing Partner, Nowak Ventures LLC, Registered Representative (Investment Banking), Wellesley, MA
- Kevin J. Scanlon, PhD, Investment Advisor, Sky Venture Group, Professor of Practice, Northeastern University, Boston MA
- Patrick Tricoli, PharmD, MBA, CEO Nanobiotix USA, Nanobiotix Corp., Cambridge MA
- Jean-Marie Vallet, PhD, MBA, Representing Launchpad Venture Group LLC, Boston MA
Companies pitching, in alphabetical order:
- Tamer Mohamed, CEO Aspect Biosystems, Vancouver, Canada: https://www.aspectbiosystems.com
- Pascal Descargues, CEO Genoskin, Toulouse FR (Liaison Office: Boston/Salem MA): https://www.genoskin.com
- Alexander Levert, CEO Osivax, Lyon Fr: www.osivax.com
- Nader Yaghoubi MD, PhD, CEO Pathmaker Neurosystems, Boston MA (Liaison Office Paris Fr): pmneuro.com
- Ann Kwong, PhD, CEO Trek Therapeutics, Cambridge MA: trektx.com
- Alain Lemproye, CEO Yposkesi Genopole, Évry Fr: www.yposkesi.com
GLOBAL ROADMAP 3 (Panel #4) focused on EU (and US) Market Entry Panelists:
- Moderator: Julius Steffen Manager, Bionest Partners, Paris FR/New York USA
- Francis Marsland, Head of Business Development, Vicarius Pharma AG, Switzerland
- Sandford D. Smith, Chairman & Founder, Global BioLink LLC.
- Rogerio Vivaldi, MD, Executive Vice-President, Bioverativ – a Sanofi Company.
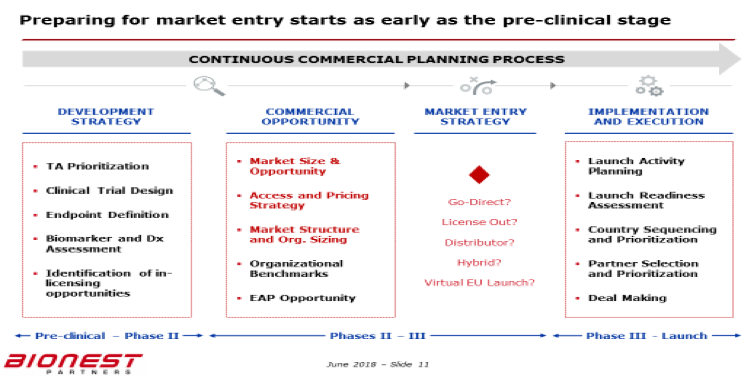
Summary Box 5– – Preparing Market Entry
[Slide courtesy of Bionest Partners]
The 2018 Organizing Committee would like to thank all the participants, the City of Boston, the Consulate General of France, our generous Sponsors (BIONEST Partners, IPSEN, MWE: McDermott Will Emery,4Clinics, Nanobiotix, CPL Physicians, as well as, Genopole & Clintec), Supporting Organizations from the Commonwealth of Massachusetts and France, and the 2018 team, including our reception desk volunteers and the Boston Exchange Conference Center.
REFERENCES:
1) Bennani Y. Drug discovery in the next decade: innovation needed ASAP.
Drug Discovery Today, June 2011.
2) Bloomberg. Ipsen says small is beautiful when scouting for new medicines.
Phil Serafino, July 10 2017.
3) Boston Globe. Robert G Urban. https://www.bostonglobe.com/business/2015/08/07/five…robert-urban/…/story.html
4) Boston Paris Biotechnology Summit 17 May 2018 Synopsis http://www.bostonbiotechnologysummit.com/ & Booklet: https://drive.google.com/open?id=0BzbkxZxp8WWjazBQUXVBRXpXb2c
5) Biotech Finances: No 813, 28 May 2018.
6) Biotech-Finances. (Dossier) Boston. No.719, 04.2016, pages 5-7.
7) Bourse (la) et La Vie.com, Shahin Gharakhanian Interview, 21-Apr-2017.
8) France Biotech & BCG: Boston Consulting Group. French Health Tech. Report, 11.2017.
9) Murcko M. What Makes a Great Medicinal Chemist? A Personal Perspective Journal of Medicinal Chemistry, Publication Date (Web): May 10, 2018 (Perspective) DOI: 10.1021/acs.jmedchem.7b01445
10) Pisano GP: Science Business. The Promise, the Reality and the Future of Biotech. Harvard Business School Press: Boston, 2006.